ISOTOPIC ANALYSIS
Isotopes are different types of atoms of the same chemical element, each having a different atomic mass. Isotopes of an element have nuclei with the same number of protons but different numbers of neutrons.
Isotope analysis is the identification of isotopic signature, the distribution of certain isotopes within chemical compounds.
Isotope ratio mass spectrometry is a specialization of mass spectrometry, in which mass spectrometric methods are used to measure the relative abundance of isotopes in a given sample.
| Parameter | Amt. Required | Precision |
|---|---|---|
| Pb - Isotopic analysis by HR-ICP-MS (Pb >15ppm) | ~ 1 g | 0.4 - 0.5% |
| Pb - Isotopic analysis | ~ 1 g | 0.1 - 0.2% |
| Nd - Isotopic Analysis | ~ 1 g | |
| Sr - Isotopic Analysis | ~ 1 g | |
| Sm-Nd - Isotopic Analysis | ~ 1 g | |
| Rb-Sr - Isotopic Analysis | ~ 1 g | |
| Hg - Isotopic Analysis | ~ 1 g | |
| δ 13 C – Graphite or Organic Material | 1 mg C | 0.2 ‰ |
| δ 13 C and δ 18 O – Carbonates | 1 mg C | 0.2 ‰ |
| δ 13 C and δ 18 O – Siderite, Magnesite, Dolomite | 1 mg C | 0.2 ‰ |
| Nitrogen Isotopes - Organic Material | ||
| Deuterium Isotopic Analysis – Water | 20 ml | 3.00% |
| Deuterium Isotopic Analysis – Minerals | 1 mg | 0.2 ‰ |
| δ 18 O – Water | 20 mL | 0.2 ‰ |
| δ 18 O – Silicates | 15 mg | 0.3 ‰ |
| δ 18 O – Sulphates | 25 mg | 0.3 ‰ |
| δ 18 O – Organics | 10 mg N | 0.2 ‰ |
| δ 34 S – Sulphate | 10 mg | 0.2 ‰ |
| δ 34 S – Sulphate in water | ||
| δ 34 S – Sulphide | 5 mg | 0.2 ‰ |
| δ 34 S – Sulphide-bearing material which contains carbonate | 100 mg | 0.2 ‰ |
| δ 34 S – Silicate rocks which contain sulphur | ||
| 3 H – Direct | 20 mL | 8.0 TU |
| 3 H – Enriched | 1L | 0.8 TU |
| 3 H – Accelerator Mass Spectrometry (AMS) | 1L | |
| C-14 - Accelerator Mass Spectrometry (AMS) | please inquire |
Deuterium isotopes are determined by injecting water samples into a Thermo Finnigan DeltaPlusXL IRMS system, coupled with an elemental analyzer. The chromium reduction tube within the elemental analyzer converts the water samples to hydrogen gas at 950°C. A helium carrier gas then transfers the hydrogen gas to the IRMS system. Hydrogen isotopic ratios are measured and deuterium isotopes are reported.
Samples weighing 0.02 to 1.0 g are wrapped in molybdenum foil and placed in a platinum crucible which is then suspended inside a quartz extraction vessel. The vessel and its contents are outgassed in a vacuum at 120°C for 4 hours to remove surface-adsorbed water. The sample is then inductively heated at 1400°C for up to 20 minutes and the gases are collected in a trap held at -196°C. Nearly all of the hydrogen is released in the form of water, but miniscule quantities of hydrocarbons or molecular hydrogen released or produced during this treatment are oxidized over CuO at 550°C to form H2O and CO2 which are also collected in the trap. The accumulated water representing the total amount of hydrogen in the samples is separated from the other gases by differential freezing techniques. The water is reacted with uranium at 900°C to produce H2 and collected on charcoal at -196°C. The volume of the H2 is measured manometrically. Analyses of the water contents are reproducible to ± 0.2 weight percent.
Isotopic analyses, made by conventional isotope ratio mass spectrometry, are reported in the familiar notation in per mil relative to the V-SMOW standard. Duplicate analyses are made of some of these samples and the δD values agree to better than ± 3. Using the procedure described above we measured a δD value of -65 for the NSB-30 biotite standard.
Oxygen isotopes ( δ 18 O) are determined by conventional CO2–H2O equilibration (Epstein and Mayeda, 1953). A small aliquot of tank CO2 is added to a vessel containing a surplus of the sample water. The vessel is sealed and emerged in a water bath at 25.0 ° C and agitated to facilitate H2O–CO2 equilibration. Equilibration time is 48 hours, long enough to equilibrate brines with high Mg contents. CO2 from equilibrated samples is extracted on a vacuum line and cryogenically purified. Isotopic analyses are performed on a Finnigan MAT Delta Gas Isotope Ratio Mass Spectrometer (GIRMS). Isotopic data are reported in the standard delta notation as per mil deviations from V–SMOW. The CO2–H2O equilibration factor used is 1.0412. External reproducibility is ±0.14‰ (1 σ ) based on repeats of our dilute U of S water standard.
Notation
δ 18 O = (18O/16Osample/18O/16OV–SMOW – 1)103
δ D = (2H/1Hsample/2H/1HV–SMOW – 1)103
References
Bigeleisen J., Perlman M.L. and Prosser H.C., 1952. Conversion of hydrogenic materials to hydrogen for isotopic analysis. Analytical Chemistry, volume 24, pp. 1356-1357.
Epstein S. and Mayeda T.K., 1953. Variations in the 18O/16O ratio in natural waters. Geochimica et Cosmochimica Acta, volume 4, p. 213.
Silicate and oxide samples are reacted with BrF5 at ~650°C in nickel bombs following the procedures described in Clayton and Mayeda (1963). The fluorination reaction converts O in the mineral(s) to O2 gas, which is subsequently converted to CO2 gas using a hot C rod. All reaction steps are quantitative. Isotopic analyses are performed on a Finnigan MAT Delta, dual inlet, isotope ratio mass spectrometer. The data are reported in the standard delta notation as per mil deviations from V–SMOW. External reproducibility is ± 0.19‰ (1 s ) based on repeat analyses of our internal white crystal standard (WCS). Our value for NBS 28 is 9.61 ±0.10‰ (1 s ).
Notation
δ18O = (18O/16Osample/18O/16OV–SMOW – 1)103
References
Clayton, R. N., and Mayeda, T., 1963. The use of bromine pentafluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochimica et Cosmochimica Acta, volume 27, pp. 47-52.
Tritium (T or 3H), a hydrogen isotope with a half-life of 12.43 years is being produced naturally by the impact of cosmic neutrons on nitrogen nuclei (cosmic ray spallation) by the reaction:
14 7 N+10n → 126C+31H
Free 3H collides with O2 to form 1H3HO via 3HO2 and enters the water cycle. Production in the upper atmosphere causes a steady state on the order of 4 to 25 tritium units (TU) in precipitation. Since 1952, tritium produced by open air thermonuclear tests has overshadowed natural production, at times by 2 or 3 orders of magnitude.
Liquid scintillation counting (LSC) is the technique used for the detection and quantification of tritium. Tritium measurements have a lower limit of detection of approximately 6 tritium units for water counted directly. Samples with a tritium content near this level are enriched approximately 15 times by electrolysis and then counted. The detection limit for enriched samples is 0.6 ± 0.8 TU.
Tritium measurements (counts per minute [cpm]) are converted directly into absolute concentrations where:
1 TU = 1-3H/1018-1H = 7.19 dpm/l = 3.26 pCi/l = 0.11815 Bq/l
The sample is processed as follows:
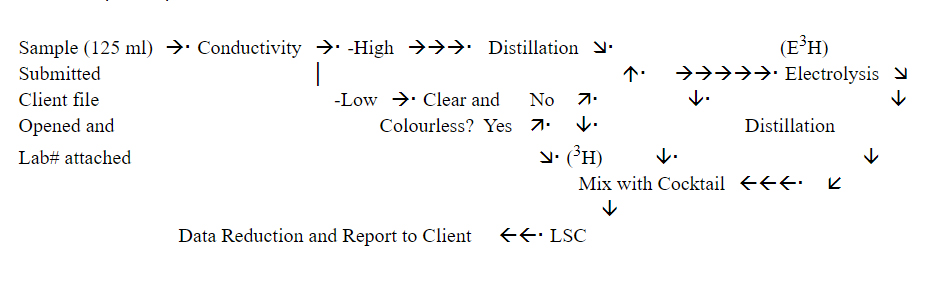
A Canberra-Packard Pico-fluor LLT (low level tritium) cocktail is used, which has a high carrying capacity for water with high efficiency and low background characteristics.
The laboratory standard is NBS-4361 tritium reference material diluted with background water which is then calibrated to NBS-4926C. The background water is from a well with Radiocarbon activity older than 3500 years and a conductivity of less than 300 µmho.
